Giving Pharma Professionals the Formula for Success
Record, track, analyze, and report your quality, safety and compliance data. All from one place.


We’re challenging the outdated notion that quality must always come at a premium. Pharma must choose smarter, more cost-effective solutions to overcome cost pressures without compromising quality. TenForce offers a compliant solution at a lower cost than traditional pharmaceutical software providers. By implementing TenForce, we’re streamlining our operations and contributing to a better cost-benefit outcome.

Bringing together quality, compliance, and safety
Elevate Quality Assurance
Minimize the risk of product defects or costly recalls by ensuring your products meet the highest safety standards.
Master Regulatory Compliance
Keep up with regulatory changes, comply with standards such as GMP and International Council for Harmonization (ICH) guidelines, and avoid costly penalties.
Prioritize Workplace Safety
Create a secure environment, safeguarding your most valuable asset – your workforce.
Optimize Financial Performance
Bring new drugs to market more quickly, reduce your cost of quality and maintain business agility in a competitive market.
Safeguard Brand Integrity
Shield your brand – protect your reputation, build trust, and maintain customer confidence.
Balancing safety, quality, and compliance - it’s no easy task for the pharma professional
With TenForce you can meet these challenges head-on. By centralizing data and streamlining processes, we help pharma professionals consistently deliver safe, high-quality products, securing regulatory compliance and upholding brand integrity.
Your prescription for operational excellence
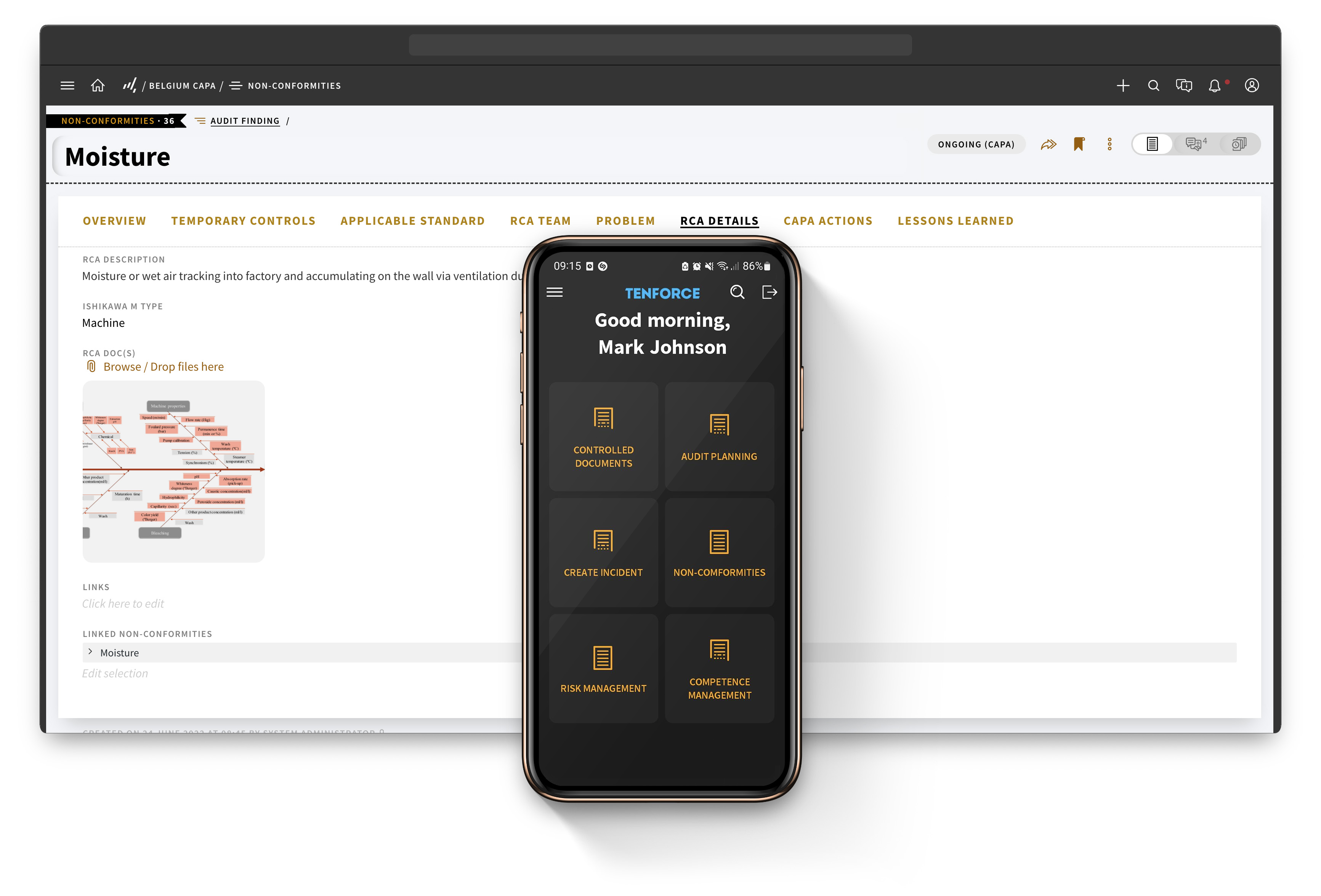
One platform for all your EHSQ data
TenForce is fully compliant with GMP Annex 11 and 21 CFR Part 11. Using TenForce helps you reduce human error, and create a secure, audit-ready environment, reducing your risk of non-compliance from the get-go.
Customer Stories
Douglas Pharmaceuticals Implement a Premier End-to-End Quality Management System
See how Douglas Pharmaceuticals consolidated all QMS processes into a single, cost-effective platform, ensuring top-notch product quality and patient safety.
Leave admin behind to focus on your quality and compliance metrics
Identify and address nonconformances swiftly
Manage customer complaints efficiently, reduce corrective action closure time, and perform thorough root-cause analysis.
Centralize quality documents and processes
Manage training, incidents, audits, changes, risks, and inspections from a centralized location.
Create efficiencies and boost productivity
Save time and enhance scalability with flexible workflows and templates for standardized processes.
Keep up to date with regulatory changes
Access ready-to-go regulatory content to keep up to date with changes and maintain compliance
Avoid disruption to your workflows
TenForce is flexible and highly configurable to your evolving requirements.
15%
increase in the identification and resolution of non-conformances
10%
improvement in customer satisfaction
TenForce is creating efficiency and transparency. It’s taking away the administrative burden. This limits the number of mistakes, immediately reducing non-compliance risks. And it creates time to perform meaningful tasks that focus on quality.

Dive deeper into this topic
Competence Management – Why Doing It Right Matters
59% of companies are still struggling to organize and complete training, even when it is required by law.1 More worrying still — companies without a proper training program are experiencing a 24% higher rate of workplace incidents than those with such programs.2
Putting the human cost of workplace incidents aside, there’s a pretty hefty price tag attached too. 15% of OSHA’s most frequently issued citations are related to employers failing to provide required training.…
How to Get More Value from Your Audit Management Efforts
Audits play a crucial role in ensuring your business operates safely and stays compliant with regulations. But here’s the real question: how can you make your audit management work even harder for you? We’ll share four essential areas to focus on for optimizing your audit management. And, to top it all off, we’ll reveal how they all come together in one comprehensive digital solution.
#1: Centralizing Your Audit DataFew things are more frustrating than searching for vital information in an endless maze of documents, emails, and legacy systems.…
CAPA Management: From Chaos to Control with EHSQ Software
Meeting environmental, health, safety, and quality (EHSQ) standards in complex industrial environments can feel overwhelming. Non-conformities have far-reaching consequences from putting safety at risk and causing environmental damage to attracting regulatory penalties and damaging your organization’s reputation…the list goes on.
But fear not, there’s a solution that can help you tackle non-conformities head-on. It’s time to implement CAPA Management (Corrective Action and Preventive Action) software.
In this blog post, we’ll address the common challenges faced during the CAPA process and uncover the key advantages of utilizing software to streamline your CAPA management. …
Putting Quality Management Centerfold
International Standards ISO 9000 and ISO 9001 describe the means and method of setting up a Quality Management System (QMS). What is not immediately obvious when reading these documents, is that they actually seek to help create a company culture to support the mission for better quality management, and not simply help to set up a QMS.
And it’s clear why: quality management no longer simply belongs to the quality assurance department or team.…
Your peers trust us
Our customers rely on TenForce to foster a proactive safety culture and drive continuous process improvement. The outcome? Increased team efficiency, reduced administrative burden, and significant cost savings due to fewer fines and incident-related downtime.





Book a demo
Curious to see how TenForce can make your workday easier? Book a demo, and we’ll show you what our EHSQ platform can do! One of our in-house experts will guide you through the ins and outs, showing you exactly how TenForce can tackle your challenges, whether it’s managing safety, streamlining compliance, or keeping quality in check.
So, choose a time that works for you, and let’s explore how TenForce fits into your day-to-day.
Ask us questions. Get clear answers. Share the recording with your colleagues.
